Medical Device Regulatory Affairs Outsourcing Market Dynamics: Key Drivers and Restraints 2031
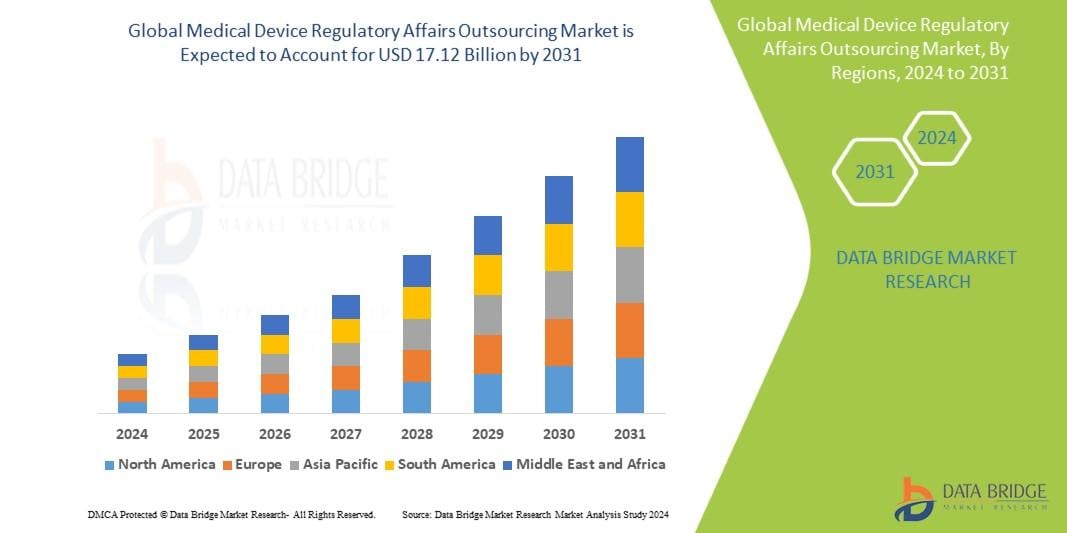
Medical Device Regulatory Affairs Outsourcing Market Growth, Demand and Forecast 2031
The Medical Device Regulatory Affairs Outsourcing Market sector is undergoing rapid transformation, with significant growth and innovations expected by 2031. In-depth market research offers a thorough analysis of market size, share, and emerging trends, providing essential insights into its expansion potential. The report explores market segmentation and definitions, emphasizing key components and growth drivers. Through the use of SWOT and PESTEL analyses, it evaluates the sector’s strengths, weaknesses, opportunities, and threats, while considering political, economic, social, technological, environmental, and legal influences. Expert evaluations of competitor strategies and recent developments shed light on geographical trends and forecast the market’s future direction, creating a solid framework for strategic planning and investment decisions.
Brief Overview of the Medical Device Regulatory Affairs Outsourcing Market:
The global Medical Device Regulatory Affairs Outsourcing Market is expected to experience substantial growth between 2024 and 2031. Starting from a steady growth rate in 2023, the market is anticipated to accelerate due to increasing strategic initiatives by key market players throughout the forecast period.
Get a Sample PDF of Report - https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-medical-device-regulatory-affairs-outsourcing-market
Which are the top companies operating in the Medical Device Regulatory Affairs Outsourcing Market?
The report profiles noticeable organizations working in the water purifier showcase and the triumphant methodologies received by them. It likewise reveals insights about the share held by each organization and their contribution to the market's extension. This Global Medical Device Regulatory Affairs Outsourcing Market report provides the information of the Top Companies in Medical Device Regulatory Affairs Outsourcing Market in the market their business strategy, financial situation etc.
Parexel International (MA) Corporation. (U.S.), North American Science Associates, LLC (U.S.), SGS Société Générale de Surveillance SA. (Switzerland), Pace (U.S.), Trilogy Writing & Consulting GmbH (Germany), Creganna (Ireland), Intertek Group plc (U.K.), WuXi AppTec (China), Charles River Laboratories (U.S.), Celestica Inc. (Canada), Freyr (U.S.), Cactus Communications (India), In.Corp Indonesia (Indonesia), Eurofins Scientific (Luxembourg), Plexus Corp. (U.S.), Sanmina Corporation (U.S.), OMRON Corporation (Japan)
Report Scope and Market Segmentation
Which are the driving factors of the Medical Device Regulatory Affairs Outsourcing Market?
The driving factors of the Medical Device Regulatory Affairs Outsourcing Market are multifaceted and crucial for its growth and development. Technological advancements play a significant role by enhancing product efficiency, reducing costs, and introducing innovative features that cater to evolving consumer demands. Rising consumer interest and demand for keyword-related products and services further fuel market expansion. Favorable economic conditions, including increased disposable incomes, enable higher consumer spending, which benefits the market. Supportive regulatory environments, with policies that provide incentives and subsidies, also encourage growth, while globalization opens new opportunities by expanding market reach and international trade.
Medical Device Regulatory Affairs Outsourcing Market - Competitive and Segmentation Analysis:
**Segments**
- Based on service, the medical device regulatory affairs outsourcing market is segmented into regulatory writing and publishing, regulatory submissions, clinical trial applications, regulatory consulting, and others. The regulatory writing and publishing segment is expected to witness significant growth due to the increasing demand for regulatory documentation services to ensure compliance with stringent regulations. Similarly, the regulatory submissions segment is anticipated to grow as companies seek assistance in preparing and submitting regulatory documents to health authorities.
- On the basis of application, the market is categorized into class I, class II, class III, and IVD medical devices. Class II medical devices are projected to dominate the market during the forecast period as they require regulatory approvals but have a lower risk compared to class III devices. The increasing adoption of class II medical devices across various healthcare settings is driving the demand for regulatory affairs outsourcing services in this segment.
- By end-user, the market is segmented into medical device manufacturers, biotechnology companies, and others. The medical device manufacturers segment is expected to hold the largest market share as these companies require regulatory expertise to navigate complex approval processes and ensure compliance with international standards. Biotechnology companies are also increasingly outsourcing regulatory affairs services to focus on core R&D activities and accelerate product commercialization.
**Market Players**
- Some of the key market players operating in the global medical device regulatory affairs outsourcing market include Ithos Global, Inc., Freyr Solutions, Jaleel Services, RQM Systems, PAREXEL International Corporation, and Weinberg & Beers Regulatory Consulting LLC. These players are focusing on strategic initiatives such as partnerships, collaborations, and acquisitions to expand their service offerings and strengthen their market presence. Additionally, technological advancements in regulatory affairs software and tools are enabling market players to enhance efficiency and quality in regulatory submissions.
- Furthermore, regulatory agencies such as the FDA and EMA are continuously updating regulations and guidelines to ensure the safety and efficacy of medical devices. Market players are actively monitoring these regulatory changes and providing tailored solutions to help clients comply with evolving requirementsThe global medical device regulatory affairs outsourcing market is experiencing significant growth and evolution driven by various market segments and key players in the industry. In terms of services, the regulatory writing and publishing segment is poised for substantial growth as companies increasingly seek regulatory documentation services to meet stringent compliance requirements. Similarly, the regulatory submissions segment is expected to witness expansion as organizations look for support in preparing and filing regulatory documents with health authorities. The focus on regulatory compliance and the complexity of approval processes are driving demand for outsourcing services in these areas.
When it comes to applications, class II medical devices hold a dominant position in the market due to the requirement for regulatory approvals coupled with lower risks compared to class III devices. The rising adoption of class II medical devices across different healthcare settings is fueling the need for regulatory affairs outsourcing services within this segment. Furthermore, the segmentation by end-user reveals that medical device manufacturers are expected to command the largest market share. These manufacturers rely on regulatory expertise to navigate approval processes and adhere to international standards, highlighting the importance of outsourcing regulatory affairs services to specialized providers.
Key market players such as Ithos Global, Inc., Freyr Solutions, and PAREXEL International Corporation are at the forefront of the global medical device regulatory affairs outsourcing market. These players are employing strategic initiatives like partnerships, collaborations, and acquisitions to broaden their service offerings and bolster their market presence. Additionally, advancements in regulatory affairs software and tools are enhancing operational efficiency and quality in regulatory submissions, enabling market players to deliver comprehensive solutions to clients.
Regulatory agencies such as the FDA and EMA play a crucial role in shaping the regulatory landscape for medical devices by introducing updates to regulations and guidelines to ensure product safety and efficacy. Market players in the outsourcing industry are actively monitoring these regulatory changes and providing tailored solutions to help clients comply with evolving requirements. The dynamic regulatory environment creates opportunities for industry players to offer innovative services that address the evolving needs of medical device companies seeking regulatory support.
In conclusion, the global medical device regulatory affairs outsourcing market is poised**Market Players:**
- Parexel International (MA) Corporation (U.S.)
- North American Science Associates, LLC (U.S.)
- SGS Société Générale de Surveillance SA (Switzerland)
- Pace (U.S.)
- Trilogy Writing & Consulting GmbH (Germany)
- Creganna (Ireland)
- Intertek Group plc (U.K.)
- WuXi AppTec (China)
- Charles River Laboratories (U.S.)
- Celestica Inc. (Canada)
- Freyr (U.S.)
- Cactus Communications (India)
- In.Corp Indonesia (Indonesia)
- Eurofins Scientific (Luxembourg)
- Plexus Corp. (U.S.)
- Sanmina Corporation (U.S.)
- OMRON Corporation (Japan)
The global medical device regulatory affairs outsourcing market is experiencing robust growth propelled by various market segments and key players in the industry. The regulatory writing and publishing segment are anticipated to flourish as companies increasingly require regulatory documentation services to adhere to stringent compliance standards. Simultaneously, the regulatory submissions segment is expected to expand as organizations seek support in the preparation and submission of regulatory documents to health authorities. The enhanced focus on regulatory compliance and the intricate nature of approval processes are fueling the demand for outsourcing services within these segments.
In terms of applications, class II medical devices are set to maintain a commanding position in the market due to their regulatory approval requirements and lower risk profiles compared to class III devices. The escalating adoption of
North America, particularly the United States, will continue to exert significant influence that cannot be overlooked. Any shifts in the United States could impact the development trajectory of the Medical Device Regulatory Affairs Outsourcing Market. The North American market is poised for substantial growth over the forecast period. The region benefits from widespread adoption of advanced technologies and the presence of major industry players, creating abundant growth opportunities.
Similarly, Europe plays a crucial role in the global Medical Device Regulatory Affairs Outsourcing Market, expected to exhibit impressive growth in CAGR from 2024 to 2031.
Explore Further Details about This Research Medical Device Regulatory Affairs Outsourcing Market Report https://www.databridgemarketresearch.com/reports/global-medical-device-regulatory-affairs-outsourcing-market
Key Benefits for Industry Participants and Stakeholders: –
- Industry drivers, trends, restraints, and opportunities are covered in the study.
- Neutral perspective on the Medical Device Regulatory Affairs Outsourcing Market scenario
- Recent industry growth and new developments
- Competitive landscape and strategies of key companies
- The Historical, current, and estimated Medical Device Regulatory Affairs Outsourcing Market size in terms of value and size
- In-depth, comprehensive analysis and forecasting of the Medical Device Regulatory Affairs Outsourcing Market
Geographically, the detailed analysis of consumption, revenue, market share and growth rate, historical data and forecast (2024-2031) of the following regions are covered in Chapters
The countries covered in the Medical Device Regulatory Affairs Outsourcing Market report are U.S., Canada, Mexico, Brazil, Argentina, Rest of South America, Germany, Italy, U.K., France, Spain, Netherlands, Belgium, Switzerland, Turkey, Russia, Rest of Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Saudi Arabia, U.A.E, South Africa, Egypt, Israel, and Rest of the Middle East and Africa
Detailed TOC of Medical Device Regulatory Affairs Outsourcing Market Insights and Forecast to 2031
Part 01: Executive Summary
Part 02: Scope Of The Report
Part 03: Research Methodology
Part 04: Medical Device Regulatory Affairs Outsourcing Market Landscape
Part 05: Pipeline Analysis
Part 06: Medical Device Regulatory Affairs Outsourcing Market Sizing
Part 07: Five Forces Analysis
Part 08: Medical Device Regulatory Affairs Outsourcing Market Segmentation
Part 09: Customer Landscape
Part 10: Regional Landscape
Part 11: Decision Framework
Part 12: Drivers And Challenges
Part 13: Medical Device Regulatory Affairs Outsourcing Market Trends
Part 14: Vendor Landscape
Part 15: Vendor Analysis
Part 16: Appendix
Browse More Reports:
Data Bridge Market Research:
Today's trends are a great way to predict future events!
Data Bridge Market Research is a market research and consulting company that stands out for its innovative and distinctive approach, as well as its unmatched resilience and integrated methods. We are dedicated to identifying the best market opportunities, and providing insightful information that will help your business thrive in the marketplace. Data Bridge offers tailored solutions to complex business challenges. This facilitates a smooth decision-making process. Data Bridge was founded in Pune in 2015. It is the product of deep wisdom and experience.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC: +653 1251 1617
Email:- [email protected]
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


