Understanding The Success Of CAR T-Cell Therapy In Multiple Myeloma
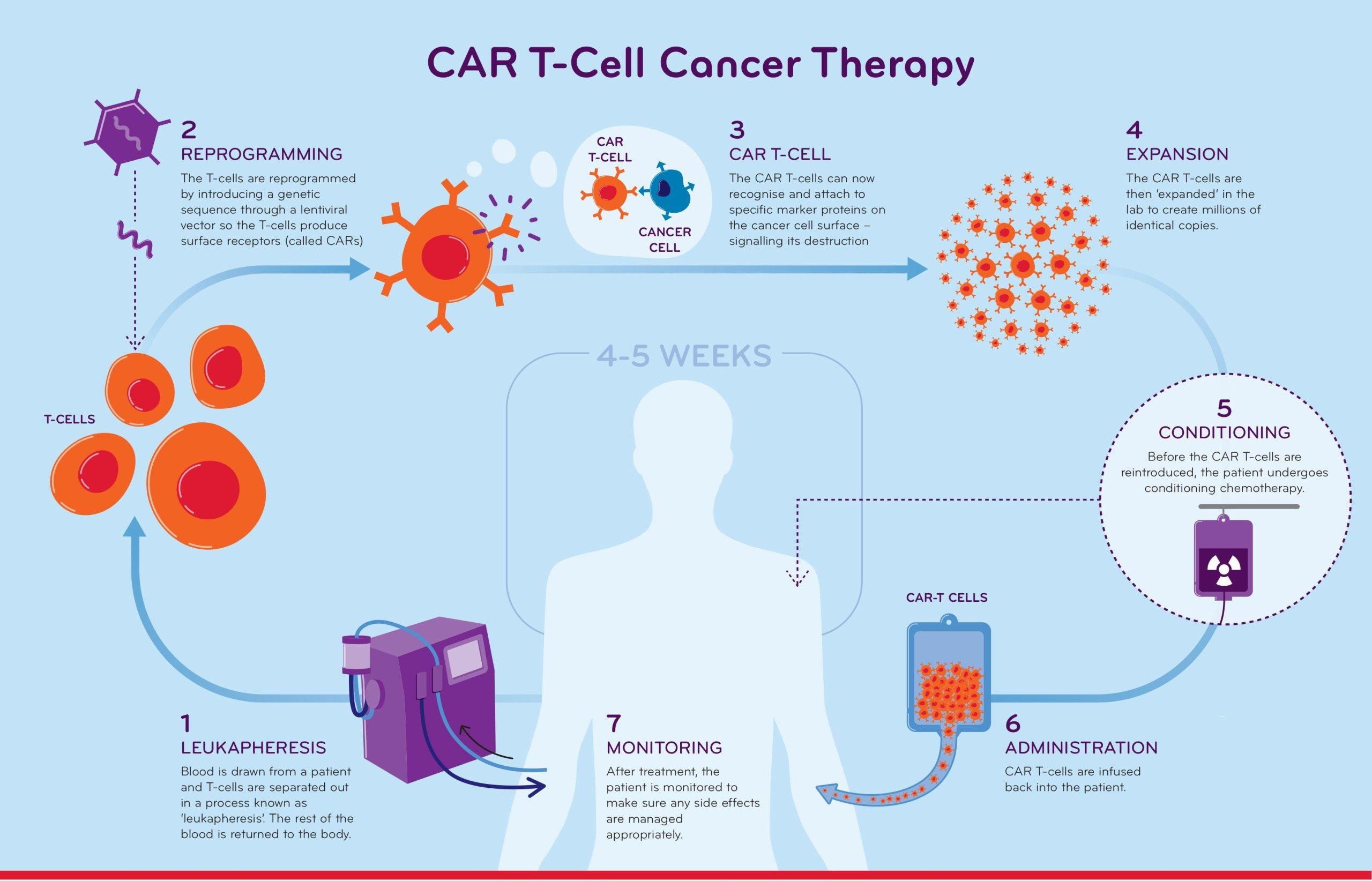
Multiple myeloma, a type of blood cancer originating in the bone marrow, remains a challenging disease to treat, particularly in cases that have become resistant to traditional therapies. Over the past decade, however, a groundbreaking treatment known as CAR T-cell therapy has emerged as a game-changer in the fight against multiple myeloma. CAR T-cell therapy for multiple myeloma also offers treatment for some patients with relapsed or refractory multiple myeloma.
Success Rates Of CAR T-Cell Therapy In Multiple Myeloma
CAR T-cell therapy for multiple myeloma has produced some remarkable results in clinical trials and real-world applications. If you take 100 patients with diffuse large B-cell lymphoma and you give them a CAR T cell as a second- or third-line treatment, 50% of those patients will be in complete remission at three months. In leukemia, it’s a different story. Around 80% to 90% of patients will go into remission, but about half of those patients will relapse within about 12 months.
-
Overall Response Rate (ORR):
Clinical studies have reported an overall response rate ranging from 70% to 90% in patients treated with CAR T-cell therapy for multiple myeloma. This means that a significant majority of patients see a reduction in tumor burden after treatment.
-
Complete Response (CR):
Achieving a complete response (CR), where there is no detectable evidence of myeloma in the body, is a major goal of treatment. Studies have shown that around 30% to 50% of patients treated with CAR T-cells can achieve a complete response.
-
Progression-Free Survival (PFS):
After CAR T-cell therapy, many patients experience progression-free survival (PFS), where the disease does not worsen for a period of time. On average, patients treated with CAR T-cells for multiple myeloma have reported progression-free survival periods of 12 to 24 months. However, some patients experience even longer remissions, with ongoing monitoring and adjustments to treatment plans as needed.
FDA-Approved CAR T-Cell Therapies: Successes and Impact
Since the approval of the first Chimeric Antigen Receptor T-cell (CAR T-cell) therapy by the U.S. Food and Drug Administration (FDA) in 2017, these therapies have revolutionized the treatment of certain types of cancer, particularly hematologic (blood) cancers. CAR T-cell therapies harness the power of a patient's own immune system to target and kill cancer cells more effectively. Below are some key FDA-approved CAR T-cell therapies, their successes, and the impact they've had on cancer treatment.
Factors Influencing Success
While CAR T-cell therapy holds great promise, its success can be influenced by several factors:
-
Patient Characteristics:
Younger patients and those with less advanced disease tend to respond better to CAR T-cell therapy. However, even in patients with heavily pre-treated or advanced disease, CAR T-cell therapy has shown significant benefits.
-
Genetic Mutations and Disease Resistance:
Some genetic mutations in the myeloma cells can make the cancer more resistant to CAR T-cell therapy. For example, high-risk genetic markers may limit the therapy's effectiveness and increase the likelihood of relapse.
-
Pre-treatment disease burden:
Patients with less aggressive or smaller disease burden may have better outcomes.
-
Age and overall health:
Older patients or those with other comorbidities may experience more complications or side effects.
-
Previous treatments:
The success of CAR-T can depend on how many lines of therapy a patient has already undergone and their response to those treatments.
The Future of CAR T-Cell Therapy After Success
The future of CAR T-cell therapy for multiple myeloma looks bright. As clinical trials continue to evolve and more data is collected, we can expect improved treatment protocols and even higher success rates. CAR T-cell therapy is already transforming the landscape of myeloma treatment, offering a viable option for patients who previously had limited choices.
For patients with relapsed or refractory multiple myeloma, CAR T-cell therapy represents a beacon of hope. With continued advancements and ongoing research, the therapy’s potential to improve outcomes and provide long-term remission is greater than ever before.
Conclusion :
CAR T-cell therapy is proving to be a powerful treatment option for multiple myeloma, especially in patients with difficult-to-treat disease. With high response rates, significant complete remissions, and potential for long-term survival, it’s no surprise that CAR T-cell therapy is being hailed as one of the most exciting advances in oncology. As more patients benefit from this innovative approach, we can expect even greater improvements in outcomes, making CAR T-cell therapy a cornerstone of multiple myeloma treatment in the years to come.
Research URL :- https://penposh.com/blogs/307998/Understanding-The-Success-Of-CAR-T-Cell-Therapy-In-Multiple
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


